Poster presented at SABCS 2025
Ex vivo cytokine profiling of live triple negative breast cancer patient specimens from core needle biopsies illustrates proof of concept to assess tumor response to immune checkpoint inhibition
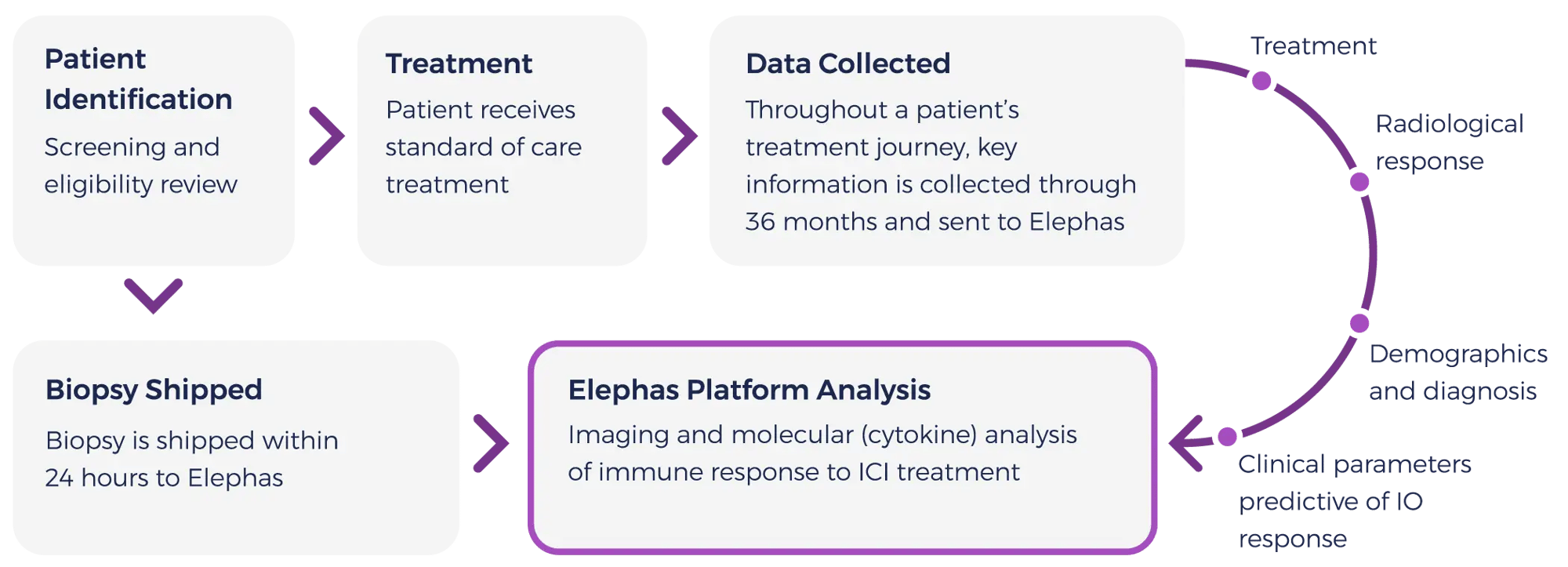
This poster provides an overview of the cytokine profiling and clinical response data from triple negative breast cancer (TNBC) patient specimens collected to date, including a closer look at a platform responder for which the patient achieved a complete response in the clinic.